Metal stains tend to show up first at edges because these areas are more exposed to moisture, debris, and surface damage that speed up corrosion. Edges often trap water, pollutants, and salts, making them prime spots for rust and patina formation. Poor drainage and thinner protective coatings also contribute. If you want to understand how to prevent these issues and protect your metal surfaces better, keep exploring these factors in more detail.
Key Takeaways
- Edges have thinner or damaged protective coatings, making them more susceptible to corrosion.
- Water and debris tend to accumulate at edges, creating ideal conditions for rust and stains.
- Sharp corners and joints trap moisture, accelerating localized metal oxidation.
- Environmental pollutants settle more easily at edges, promoting early stain formation.
- Proper drainage and protective coatings at edges can reduce initial corrosion and staining.
The Role of Metal Composition in Stain Development
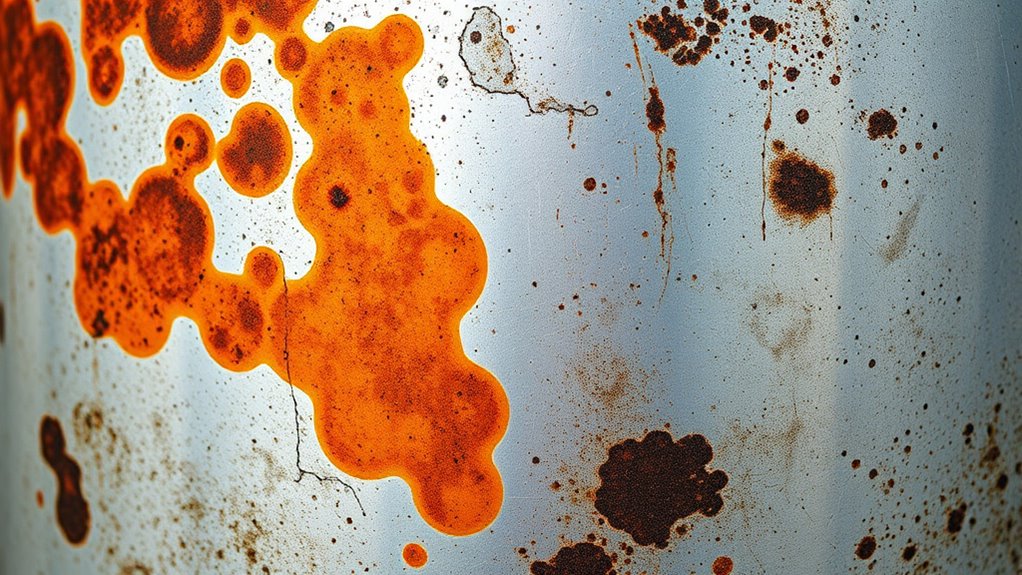
Metal composition plays a crucial role in how stains develop at the edge of surfaces. Different metals have varying tendencies to oxidize or corrode, depending on their chemical makeup. For example, iron-rich metals are prone to rust, which appears as reddish-brown stains, especially at edges where moisture can accumulate. Copper and its alloys tend to develop greenish patinas over time due to the formation of copper salts. Aluminum and stainless steel are more resistant, but their edges might still show staining if exposed to certain environmental conditions. Your understanding of a metal’s composition can help predict where stains might appear first and how quickly they will form. Recognizing these differences is key to preventing or managing edge staining effectively.
How Environmental Factors Accelerate Edge Corrosion

Environmental factors play a significant role in speeding up edge corrosion, especially when conditions promote moisture and chemical interactions. Humidity, rain, and condensation create a wet environment that accelerates rust formation at edges, where protective coatings are often thinner or damaged. Salt, pollutants, and acids in the air or water further intensify corrosion by reacting with metal surfaces, breaking down protective layers. Temperature fluctuations cause expansion and contraction, leading to cracks and gaps that allow moisture ingress. Windborne debris and chemical particles settle at edges, increasing exposure. These factors work together to weaken the metal’s surface, making edges more vulnerable to rapid corrosion. Additionally, the presence of electric dirt bikes and other powered equipment nearby can contribute to environmental wear through increased chemical exposure and physical vibrations that may damage protective coatings. By understanding how environmental conditions promote edge deterioration, you can better protect your metal assets from premature damage.
The Impact of Surface Geometry and Material Design

Surface geometry and material design markedly influence how and where corrosion initiates and spreads. Sharp edges, corners, and uneven surfaces create spots where protective layers are thinner or broken, making these areas more vulnerable to moisture and oxygen penetration. You’ll notice corrosion often starts at these points because they trap water or debris, fostering localized rust. Similarly, material choices matter; metals with higher galvanic potential or poor corrosion resistance are more likely to stain first at edges or joints. Design features like overlapping joints or exposed seams can also accelerate edge corrosion. By understanding how surface shape and construction influence corrosion, you can better predict problem areas, implement protective measures, and extend the lifespan of your metal components. Recognizing corrosion-prone areas allows for targeted maintenance and improved material selection.
Common Causes of Metal Stains Appearing First at the Edges

Edges and corners are naturally more vulnerable to stains because they tend to trap moisture and debris, creating prime conditions for corrosion to start. This makes them the first spots where metal stains often appear. Several common causes contribute to this pattern:
- Poor drainage: Water pools at edges, prolonging exposure to moisture and accelerating oxidation.
- Surface damage: Chips or scratches at edges expose fresh metal, making it more prone to corrosion.
- Environmental factors: Salt, pollutants, or humidity tend to settle at edges, intensifying corrosion risks.
These factors combine, making edges the prime locations for early staining. Recognizing these causes helps you better understand why stains show up first at the edges of your metal surfaces.
Strategies to Prevent and Minimize Edge Metal Stains

To effectively reduce edge metal stains, you should focus on implementing targeted protective measures that address the vulnerabilities specific to these areas. Start by applying a high-quality sealant or protective coating along the edges, creating a barrier against moisture and corrosive elements. Regularly inspect and maintain these coatings to guarantee they stay intact and effective. Consider installing edge trims or metal strips that act as physical barriers, preventing direct contact with liquids or corrosive substances. Proper drainage is essential; ensure water flows away from edges rather than pooling. Additionally, avoid harsh cleaners or abrasive tools on edges that could damage protective layers. By actively safeguarding these vulnerable spots, you minimize exposure and greatly reduce the likelihood of early metal staining at the edges. Consulting water quality and flow rate guidelines can also help identify potential issues before staining occurs.
Frequently Asked Questions
Can Metal Stains Be Completely Removed Once They Appear?
Yes, metal stains can often be completely removed if you act promptly. You should use a specialized metal stain remover or a mixture of vinegar and baking soda, applying it directly to the stained area. For stubborn stains, gentle scrubbing with a soft brush can help. However, some stains may be difficult to eliminate entirely if they’re deeply embedded or if the metal has reacted strongly with the surface.
Do Certain Metals Inherently Stain More Than Others?
Absolutely, some metals are the divas of staining—copper, iron, and nickel, for instance, love to leave their colorful fingerprints, turning your surfaces into abstract art. These metals inherently stain more because they oxidize easily or react with moisture, creating those stubborn, vibrant marks. So, if you’re aiming for a pristine look, steer clear of these troublemakers or be prepared for a colorful, albeit unwanted, masterpiece.
How Long Does It Typically Take for Stains to Develop at Edges?
Stains at the edges typically develop within a few days to a week, depending on the metal and exposure conditions. If you notice staining quickly, it’s often due to moisture or air contact accelerating oxidation. To prevent this, keep the area dry and clean, and consider applying protective coatings. Regular inspection helps catch stains early, minimizing long-term damage and maintaining your metal’s appearance.
Are There Specific Coatings That Prevent Edge Staining Effectively?
Yes, you can use specific coatings like epoxy or polyurethane to prevent edge staining effectively. These coatings create a strong barrier that stops moisture and metal ions from reaching the surface, reducing staining risks. Make sure to apply the coating thoroughly, especially at edges and corners, for best protection. Regular maintenance and reapplication as needed will keep your surfaces resistant to metal stains over time.
Do Environmental Conditions Influence the Color or Severity of Stains?
Environmental conditions definitely influence both the color and severity of metal stains. Humidity, temperature fluctuations, and exposure to salt or pollutants can accelerate staining and alter its appearance. You might notice darker, more prominent stains in humid or salty environments, while dry conditions may cause less intense discoloration. To minimize these effects, consider protective coatings and regular maintenance, especially if you’re in areas with harsh environmental factors.
Conclusion
So, next time you notice those early edge stains, remember it’s no coincidence—they’re often the first whispers of metal reacting to its environment. By understanding how composition, design, and surroundings play a role, you can better protect your surfaces. Sometimes, a simple change in how you care for or position your materials can make all the difference. After all, in the dance of metal and moisture, it’s those edges that lead the way—so stay vigilant.









